Introduction
Anode and cathode are terms used in chemistry, physics, and electronics. It turns out that there is some confusion regarding the electrical potentials of anodes and cathodes. For example, in electronics, the anode is typically called "positive" and the cathode as "negative" terminal. In electrochemical processes, "it depends"; for instance, from the perspective of battery manufacturers, it's precisely the opposite, and in the case of a rechargeable battery, it depends on whether it's being charged or discharged.
This article discusses the electrical potential of anodes and cathodes and how to remember when dealing with anode versus cathode.
Anode, Cathode - Etymology
The terms anode and cathode originate from the Greek language - ἄνοδος (anodos) means ascent, going up, and κάθοδος (kathodos) means descent, going down. It makes sense from an electrical standpoint:
- The electrical potential of the anode "the way upward" - becomes more positive.
- The potential of the cathode "the way downward" - becomes more negative.
The words "anode," "cathode," and "ion" were introduced into scientific vocabulary by William Whewell[1] while collaborating with the renowned Michael Faraday.
Anode, Cathode - Definition
The most common definition of anode and cathode comes from electrochemistry and is formulated as follows:
- The anode is the electrode where the oxidation reaction occurs.
- The cathode is the electrode where the reduction reaction occurs.
Oxidation and Reduction
Unfortunately, most electricians and electronics enthusiasts do not know (or do not remember) what oxidation or reduction means in chemistry. By applying some simplification, we can explain these concepts as follows:
- The process of oxidation is realted to the loss of electrons by the substance undergoing oxidation. If the substance is a metal electrode that is "losing" electrons, then the charge of that metal becomes more positive, i.e., it "increases." Oxidation of the metal raises its electrical potential (the potential "goes up"), making the electrode of that metal an anode.
- The process of reduction is realted to the gain of electrons by the substance. If the metal electrode receives electrons, its electrical potential becomes more negative (e.g., a positively charged metal ion becomes a neutrally charged atom). Therefore, the electrical potential of the metal electrode "descends," making it a cathode.
For electronics engineers
For electricians and electronics engineers, the following rule will be more straightforward to remember:
Anode
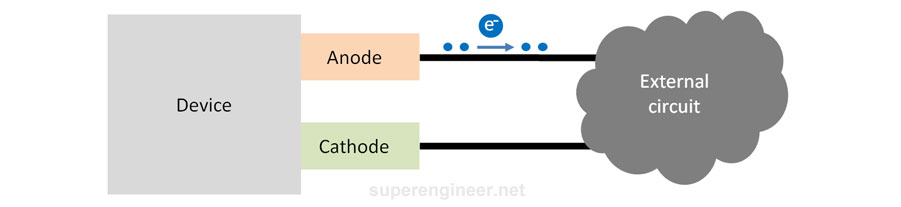
The anode is the device electrode that releases electrons into the external circuit[2]. Electrons "flow out of the device through the anode", so due to the loss of electrons, the anode's electrical potential increases, becoming more "positive".
Cathode
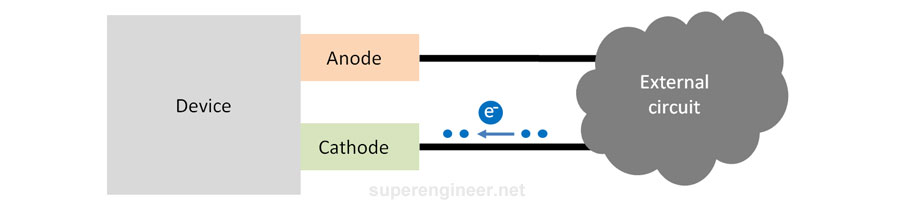
The cathode is the device electrode that receives electrons from the external circuit[3]. Electrons "flow into the device through the cathode", so the electrical potential of the cathode becomes more "negative".
Anode and Cathode - Examples
Below are examples of devices where the anode and cathode have different electrical potentials. What always remains constant is the principle that the anode releases electrons (oxidation), and the cathode receives electrons (reduction). Thus, the principle from the definition is fully preserved.
Electrochemical Cell
An electrochemical cell is a general term for a device containing two electrodes immersed in an electrolyte. Electrochemical cells are divided into galvanic cells and electrolytic cells.
Galvanic Cell
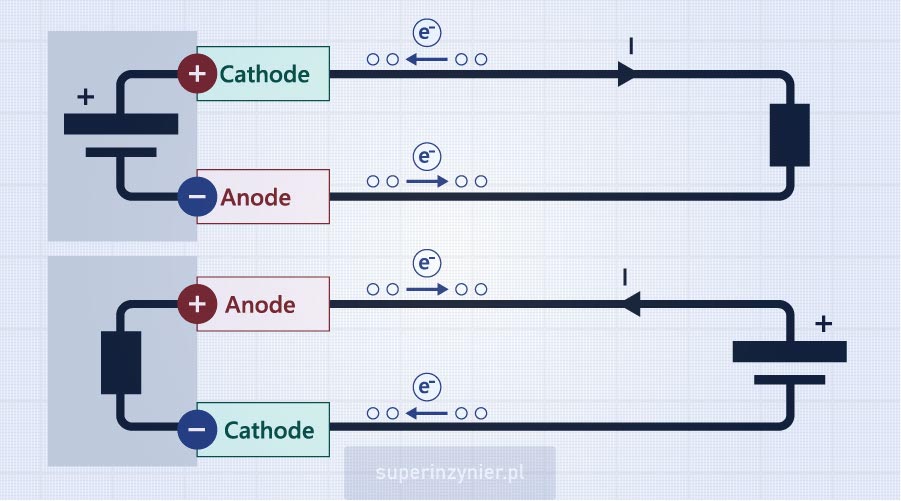
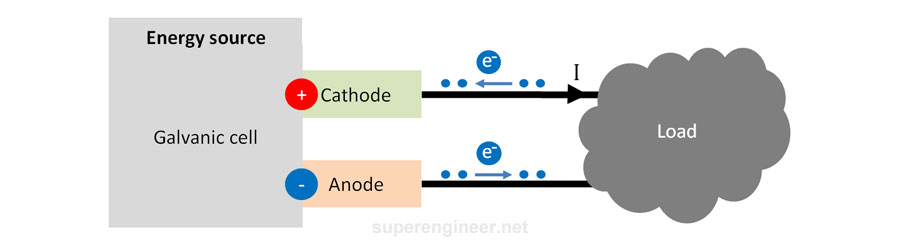
A galvanic cell is a source of electrical energy. It is a single battery cell. As seen in the figure below, electrons flow out of the "negative" terminal of the cell towards the load and then flow into the "positive" terminal of the cell. The electrode that receives electrons is the cathode (+), and the electrode that releases electrons is the anode (-).
Electrolytic Cell
An electrolytic cell is a receiver of electrical energy. An external power source forces current flow in this cell. In the figure below, electrons from the power source (e.g., battery) flow into the lower electrode (-) and then out of the upper electrode (+) towards the positive terminal of the power source. Thus, the lower electrode "receives electrons," becoming more negative (potential decreases), and the upper electrode "releases electrons," becoming more positive (potential increases). Therefore, the upper electrode (+) is the anode, and the lower electrode (-) is the cathode.
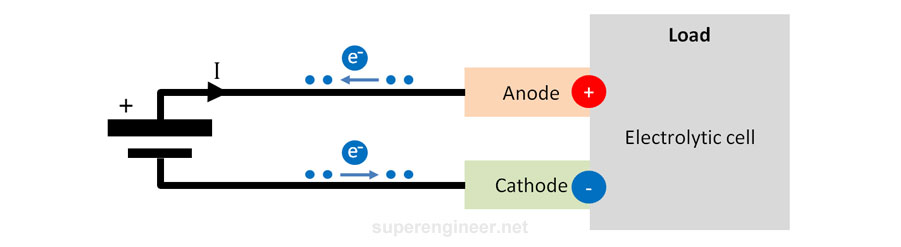
Electrolytic cells are used, among other things, in electroplating processes. This method is used to apply certain coatings to connector contacts to reduce the risk of corrosion. Galvanic coatings are very popular and are used in almost every electronic component.
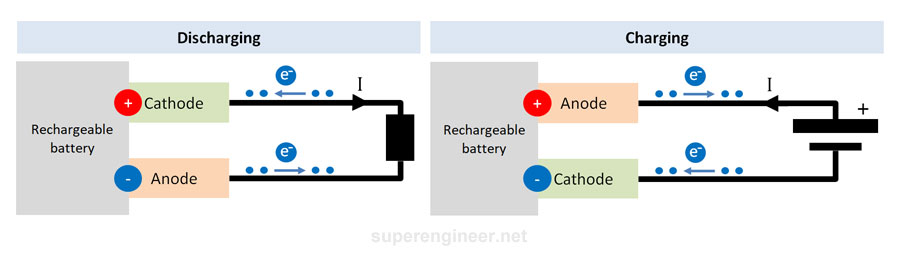
Rechargeable battery
A rechargeable battery is a source of electrical energy just like a standard battery. The difference is that a rechargeable battery is a type of galvanic cell that can be recharged. Depending on the mode of operation (charging or discharging), a particular electrode of the device is the anode or cathode. The figure below shows the terminals of the device during charging or discharging, indicating which terminal is the anode and which is the cathode.
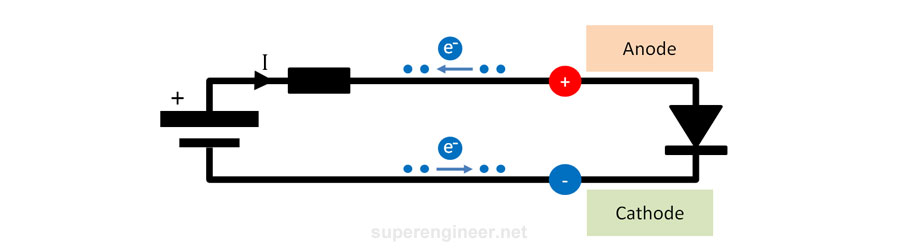
Semiconductor Diode
A semiconductor diode has two terminals: anode and cathode :). The electrical symbol is shown in the figure below. The cathode is the electrode to which electrons flow during diode forward bias, and the anode is the electrode from which electrons flow (during forward bias). So, the anode electrode is "positive", and the cathode electrode is "negative".
Vacuum Tube
Vacuum tubes are glass bulbs with metal electrodes inside. Depending on the type of tube, we have diodes (two electrodes - anode and cathode), triodes (3 electrodes), tetrodes (4 electrodes), pentodes (5 electrodes), etc. Every tube has a cathode and an anode. Additional electrodes are called grids (controlling electron flow). Usually, the cathode may be heated (the tube has a "filament"). A vacuum tube is a receiver, so the terms anode and cathode have the same meaning as in a semiconductor diode.
Vacuum tubes were popular before the introduction of semiconductors. They are currently used in some professional solutions, such as high-end audio amplifiers.
Cathode Ray Tube (CRT)
A cathode-ray tube (CRT) is a type of vacuum tube that contains one or sometimes more electron guns (cathode). These guns emit electron beams, which are controlled to produce images on a phosphorescent screen. The anode and cathode are understood in the same way as in typical electron vacuum tubes.
Electrochemical Corrosion
Electrochemical corrosion is a phenomenon that involves a flow of current between anode and cathode in an electrolyte environment. Read more about this effect in the article: Corrosion of electrical connectors.
Electrochemical Migration
Electrochemical migration is a phenomenon involving an electrolytic cell. The article Dendrites and Corrosion provides more information.
Conclusion
Anode and cathode are device terminals whose electrical potential can be "negative" or "positive" depending on whether the device is a source of energy (galvanic cell) or a receiver of energy. This aspect can lead to a series of misunderstandings, sometimes very costly.
The terms anode and cathode should be used cautiously, especially in electrochemical processes. If there is any doubt about the labeling of electrodes, it is better to use the designation [+] and [-], or to make sure exactly which electrode is the anode and which is the cathode.
References
- https://www.britannica.com/biography/William-Whewell
- https://www.britannica.com/technology/anode
- https://www.britannica.com/technology/cathode