Introduction
The subject of this article is a phenomenon known as electrochemical migration (ECM). It is a form of corrosion found in electronic devices, and its visible result is called a dendrite.
Electrochemical migration is a cause of failure in electronic devices, especially those operating in high humidity environments.
Corrosion
Corrosion is "the process of destruction (degradation) of materials as a result of chemical or electrochemical reactions occurring at the interface with the surrounding environment"[1].
Depending on the type of the material in question and the environment with which it comes into contact, the corrosion phenomenon can be based on various mechanisms: chemical, electrochemical, mechanical (friction), microbiological, etc.
Electrochemical corrosion
Electrochemical corrosion (ECC) is the destruction of the surface of a metal due to the presence of the differential electric potential between areas of the metal which are in an electrolyte environment. The electrolyte is usually water, which contains various ions that allow current to flow.
In this phenomenon, the metal with a higher potential is an anode(+), so the metal with a lower potential will be a cathode(-). The atoms of the metal that lose electrons are released from the the anode(+), resulting in the loss of the anode's material. We can therefore conclude that "the anode corrodes". During this time, an electric charge (current) flows through the electrolyte between the anode and the cathode[2].
Electrochemical corrosion can occur on the surface of the metal, where so-called "micro-cells" are formed, or between different metal objects, in which case so-called "macro-cells" are formed.
- Micro-cells. The surface of metals is not perfectly homogeneous, there are various impurities and inclusions of other elements, etc. Metals have a grain-like (crystalline) microstructure. The boundaries of the grains relative to the interior of these grains have different energy levels. If such a grainy surface is covered with an electrolyte (e.g., salt water), a "microcell" is formed and the process of electrochemical corrosion begins. The grain boundary will then be the anode(+), and the interior of the grain will be the cathode(-).
- Macro-cells. They are formed when two different metals come into contact in an electrolyte environment. Such a macro cell would be, for example, an aluminum washer and a brass nut. Among other things, corrosion of electrical connectors can be the result of mating different contact finish materials.
Electrochemical corrosion can occur in various forms, such as: general corrosion (of the entire surface), galvanic corrosion (caused by contact between different metals), crevice corrosion (in the crevices of metals), etc.
Electrochemical migration
Electrochemical migration (ECM) is a phenomenon very similar to typical electrochemical corrosion. The difference is that the metal surfaces (anode and cathode) are electrically isolated from each other. In this phenomenon, there is a release and movement (migration) of metal ions under the influence of an electric field in the electrolyte environment. As a result, a dendrite is formed connecting the anode and the cathode.
Electrochemical migration can only occur when the following factors are present simultaneously:
- Metal electrodes. An assembled electronic device has many different metal areas. Closely spaced component leads, SMT/THT solder lands, vias, copper tracks, etc.
- Electric field. The difference of electrical potential or, in other words, the voltage between the metal electrodes during operation of the device. An electrode with a positive potential is the anode(+), while an electrode with a negative potential is the cathode(-).
- Water. The source of the water/moisture can be a high ambient humidity, water condensation effect or pouring of a conductive liquid over the product.
- Ions. Typical sources of such ions come from manufacturing processes. The most common are: active flux residues, salt, sweat from fingerprints. In addition, water itself in the surrounding environment contains a number of ions (after all, it is not a distilled water).
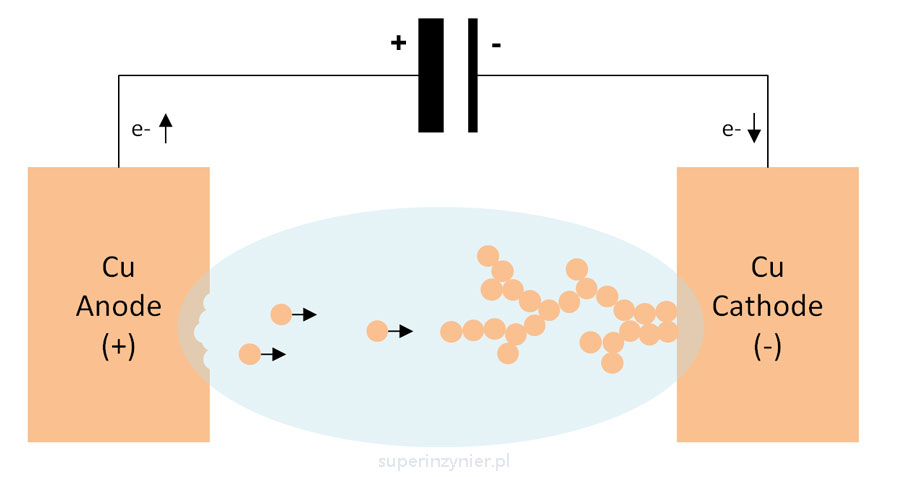
During the formation of the dendrite, the surface resistance between the anode and the cathode decreases. Eventually, the dendrite may electrically connect the two electrodes.
Dendrite
The following are example images of dendrites from various damage analyses performed:


J-STD-001 Section 8
Electrochemical migration in electronic devices can be caused by ionic residues left on the circuit board after soldering processes. Usually these are flux residues, salt from fingerprints and other deposits.
Measurement of ionic cleanliness levels over the years has been based on the R.O.S.E. test (IPC-TM-650 method 2.3.25) along with a limit of 1.56ugNaCl/cm2 for ROL1 and ROL0 class fluxes. For other types of fluxes, a corresponding limit had to be set. It is worth knowing that this level was developed in the 1970s and made sense for processes based on fluxes of the time and the fact that PCBs were washed after soldering.
The introduction of "no-clean" fluxes and the reduction of boards washing have made the 1.56ugNaCl/cm2 limit irrelevant in many cases. Finally, in 2020, the IPC organization changed the requirements related to ionic cleanliness qualification, as described in J-STD-001 Rev.H in Chapter 8.
In general: the limit of 1.56ugNaCl/cm2 was removed and the principle of "objective evidence" was introduced[4].
Protection from corrosion
The electrochemical migration can be significantly reduced by using various solutions. Depending on the application of the device, the environment in which it operates and the expected time of reliable operation, we can use one or more of the following solutions:
- Conformal coating. Conformal coating reduces the negative effects of moisture, but it is not an ideal solution. Moisture will eventually penetrate the coating. The reliability of this solution depends on the type, thickness and adhesion of the conformal coating. Be especially careful of coating dripping from the sharp edges of component leads. This is a common cause of problems.
- Underfil. Filling the gaps between the PCB and the underside of the components reduces the influence of moisture. The solution most often found in BGA assembly. Of course, underfil is very important for the mechanical resistance of soldered connections (significantly extends their life).
- Resin potting. Potting the device with a resin can eliminate the influence of moisture in many applications. However, there are sensitive areas where moisture can penetrate along the walls of connectors, cables, etc. into the product. In addition, resin can damage solder joints due to CTE differences and temperature changes.
- PCBA washing. Washing the board is designed to remove contaminants such as flux, solder balls, fingerprints, etc. Washing greatly improves the cleanliness of the board, but it is not always an easy process to control. Removing contaminants from under QFN chips can be very difficult. It is also worth remembering that not all components can be washed.
- Plasma treatment. Washing of PCB assemblies does not provide 100% assurance that all contaminants will be removed. There is a risk of leaving minor contamination. In such cases, plasma (ionized gas) can be considered. This solution removes small residues from the surface and additionally activates PCB surface, which improves varnish/resin adhesion.
- Appropriate PCB layout. There are many interesting methods to reduce the risk of migration. The simplest technique is, of course, to maximize the distance between the component leads, mask the vias, etc. More advanced techniques are a topic for another article or training :)
Summary
Electrochemical migration is one of the most difficult factors to analyze in electronic devices and is the result of several factors occurring at once. Some of them are unavoidable (i.e. electrical voltage, metal leads/fields), but the rest can be reduced through appropriate design and proper manufacturing processes.
There may be many causes in the soldering process itself. This may be due to the use of too active flux, incorrect rework, incorrectly selected SMT paste, incorrect (too low) reflow profile or wave soldering profile. Other typical factors include contamination on the PCB such as fingerprints, residues from dirty tools or from soldering frames, etc.
It is also worth familiarizing yourself with the changes in chapter 8 of the IPC J-STD-001 standard. The concept of ionic cleanliness is a topic that requires much more study and work than we think.. ;)
Footnotes
- https://encyklopedia.pwn.pl/haslo/korozja;3925930.html
- https://www.mdpi.com/2075-4701/10/10/1276
- https://www.objectiveevidence.org/ecm-explained
- IPC J-STD-001H




