Introduction
Lead-free alloys have dominated soldering processes in the electronics industry for good. The shift from lead to lead-free assemblies is the result of regulatory changes, such as the EU RoHS directive. It is worth remembering that lead alloys in soldering are still used, however, to a limited extent. This article presents the main aspects of lead-free alloys in electronics soldering.
Types of lead-free alloys
Lead-free alloys in the electronics industry are specified in EN ISO 9453 and J-STD-006, among other standards. These alloys differ in their composition, in which the main ingredient is tin (Sn) doped with elements such as copper (Cu), silver (Ag), bismuth (Bi), nickel (Ni) and others. The goal is to achieve certain melting temperatures, suitable mechanical properties and affordable prices.
Alloys are available in various forms: wires, flat bars and solder pastes for SMT soldering.
Selected lead-free alloys that we can most often find in electronics are:
Tin, silver, copper
Alloys consisting of tin, silver and copper are very popular materials. These alloys are often referred to as "SAC", which is short for the symbols of the elements used SnAgCu. Currently (2023), a very popular alloy is SAC305 (Sn96.5Ag3.0Cu0.5), which has a melting temperature of 217-220C.[1] Other less popular variants are mainly SAC405 and SAC387.
Interesting fact: SAC387 alloy is "nearly eutectic"[2] with a melting point of 217C[1,2]. This solder is recommended for high reliability applications. Ideal for wave soldering, it fills THT holes very well and leaves shiny solder joints. Of course, it is more expensive than SAC305 due to the higher amount of silver, i.e. 3.8% in SAC387 compared to 3% in SAC305.
Tin, copper, silver, other
Low-silver alloys, referred to as "Low-SAC" or "SCA" (SnCuAg), are a cheaper material than traditional SAC305. The disadvantage is the lower mechanical strength of the formed solder joints. This type of solder can therefore be used in standard commercial products where there are no special reliability requirements.
A typical alloy in this category is Sn99Cu0.7Ag0.3. This saloy is a cheaper alternative to SAC305, as it contains only 0.3% silver (SAC305 contains 3% silver). The melting temperature is 217-227C.[1]
We can also use the Alpha alloy SACX0307 (Sn-Ag0.3Cu0.7). SACX0307 contains a small doping of other elements, which are represented by the letter "X" in the alloy's name. These dopants reduce the oxidation of tin and improve the fluidity of the alloy, making its mechanical properties closer to the classic SAC305 alloy.
Tin, copper
Tin-copper alloys. An example of a solder is Sn99.3Cu0.7, in which the copper content is 0.7%. The melting temperature is 227C, so it is a eutectic alloy.[1,2]
Alloys in this group may have a greater tendency to dissolve copper from component and PCB leads (copper disolution), especially when used at the upper ranges of recommended soldering temperatures.
SN100C
SN100C (Sn99.25Cu0.7Ni0.05Ge) is a eutectic alloy with a melting point of 227C. It contains tin, 0.7% copper, 0.05% nickel and 0.01% germanium. It is characterized by shiny solder joints similar to tin-lead joints. It has low copper dissolution during the soldering process and is more mechanically robust than lead alloys or SAC305.[3]
The addition of nickel increases the fluidity of the alloy and reduces the tendency to form solder bridges. The addition of germanium improves the properties of the solder so that excess solder drains more easily from the solder joint into the bath, further reducing the occurrence of solder bridges. In addition, germanium reduces the formation of tin dross and facilitates wetting.[4]
SN100C was developed and patented by Nihon Superior and has been used in the industry since 1999. Currently, the patent rights to this material are expiring, so replacements are emerging. SN100C can be mixed with SAC305 during manual touch-ups, etc., without compromising the reliability of solder joints.[3]
Tin, bismuth, copper, nickel
These are high-quality alloys. The addition of bismuth (Bi) in small amounts increases the fatigue strength of the solder. In addition, the alloys can be doped with germanium (Ge), which reduces the oxidation of tin.
An example alloy is SN100CV (Sn-Bi1.5Cu0.7Ni) patented by Nihon Superior and offered by Balver Zinn. According to the manufacturer, the fatigue strength of this solder is about 30% higher compared to "ordinary" SN100C and exceeds that of the popular SAC305 alloy.[5]
Eutectic alloys
The name "eutectic" comes from the Greek "eutectos", which we understand as "easily fusible".
A eutectic alloy is a mixture of metals in which the melting and solidification temperatures are the same. At the same time, it is the lowest melting point of a given alloy.
The figure below shows a tin-silver-copper (Sn-Ag-Cu) phase diagram, which marks the point where the alloy composition is eutectic. The alloy is eutectic according to tests, containing 3.5%Ag +/- 0.3%Ag and 0.9%Cu +/-0.2%Cu[6], then narrowed to 3.57%Ag +/-0.05%Ag, 0.9%Cu +/- 0.04%Cu[7], and a melting point of 217.2C +/- 0.2C.[6,7]
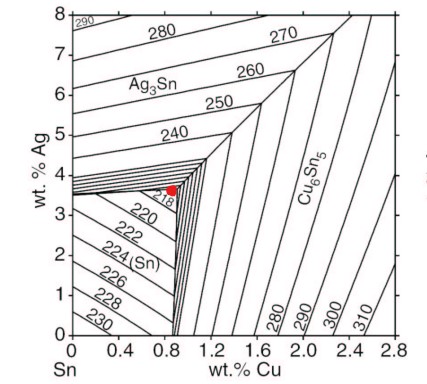
Alloy SAC387 (Sn95.5Ag3.8Cu0.7) is quite close to the confirmed eutectic point, so it is "nearly eutectic", as defined by the J-STD-006 standard.[2]
Appearance
Compared to lead solder, most lead-free alloys have a distinctive appearance, usually less shiny and with a higher wetting angle. This visual difference can affect the inspection of solder joints in the electronics industry. Depending on the type of lead-free alloy and the solidification conditions, the surface appearance can vary significantly.
The wetting angle was discussed in an article on the basics of soldering in electronics.
In the case of lead-free alloys, phenomena such as hot tear/shrink hole and filet lift can occur. These are acceptable conditions according to IPC-A-610.
What is an acceptable condition according to IPC standards? Learn about the concept of conditions in IPC standards.
Impurities in the alloy
Lead-free alloys can become contaminated with other elements, which can change their mechanical properties and melting temperatures. Impurities can come from a variety of sources, such as soldering equipment or soldered components. It is very important to monitor the level of impurities in order to maintain correct solder properties. Industry standards specify maximum allowable impurity levels for each type of lead-free alloy.
A typical problem is the increase in copper levels, which is caused by the dissolution of this element from OSP-coated PCBs and the tinning of copper wires. Increasing the amount of copper in SAC alloys causes an elevation of its melting temperature, which makes the soldering process much more difficult.
Reducing excess copper in solder involves periodically adding a special copper-free alloy, such as SAC300, to the bath. This action keeps the level of this element within specified limits. Regardless of the addition of SAC300, the composition of the alloy in the soldering bath should be periodically checked for impurities.
Regulatory compliance
The use of lead-free alloys in soldering electronic devices is related to the ban on lead and other hazardous elements. Examples of more important regulations:
RoHS in the European Union
Directive 2002/95/EC on the Restriction of Hazardous Substances (RoHS) came into force on July 1, 2006. It introduced restrictions on the use of lead and other elements, mainly in consumer electronics. Then on July 21, 2011, Directive 2011/65/EU, referred to as RoHS2, came into force. The new regulation expanded the scope of covered products and added new obligations for manufacturers in terms of declaration of conformity and product labeling.
RoHS in China
China has its own version of RoHS, known as China RoHS. As of July 1, 2016, a new version known as China RoHS2 is in effect.
USA
The United States has no federal regulations restricting the use of lead in electronics (as of 2023). However, some states, such as California, have their own regulations limiting the use of lead in electronics.
Advantages of lead-free soldering
- Significant reduction in the negative impact of soldering on the health of workers performing the process.
- The absence of lead eliminates its penetration into the soil and groundwater. This is of considerable importance for the well-being of humanity and the environment as a whole.
Disadvantages of lead-free soldering
- Higher alloy cost, especially for a high silver content solder.
- The higher temperature of the soldering process increases the stress on electronic components, which can shorten their service life. In general, the vast majority of components are now suitable for soldering at elevated temperatures, so this aspect is no longer as critical as in previous years.
- Higher electricity costs due to higher soldering temperatures.
- Faster deterioration of soldering equipment.
- Risk of tin whisker formation.
Summary
Lead-free soldering has become an industry standard due to its compliance with environmental regulations and health benefits. The market offers many types of lead-free alloys, whose melting temperatures and mechanical parameters can vary significantly. Choosing the right alloy is therefore crucial to the cost of the process and the reliability of the achieved solder joints.
In addition to the appropriate alloy, you must ensure the right soldering flux. For reflow soldering, it's important to use the best SMT soldering profile. For wave soldering, it's important to use the right THT soldering profile.
Footnotes
- EN ISO 9453:2020
- IPC J-STD-006
- https://aimsolder.com/sites/default/files/sn100c-sell-sheet.pdf
- https://aimsolder.com/sites/default/files/the_effect_of_germanium_additions_on_sn100c.pdf
- https://www.balverzinn.com/en/lote-reader/sn100cv-en.html
- K.Moon, ... ,Experimental and Thermodynamic Assessment of Sn-Ag-Cu Solder Alloys, JEM no.29, 2000
- https://www.nist.gov/publications/ternary-eutectic-sn-ag-cu-solder-alloys

 PL
PL  EN
EN 
