Introduction
The soldering process requires proper preparation of the surfaces to be joined before contact with the liquid solder. These surfaces should be clean and free of metal oxides, and this is the role of the flux. The following article addresses issues such as performance, classification, activity level and other important characteristics of the fluxes.
Metal oxides
The surfaces of most metals are covered with a layer of oxides, which is the natural result of contact with oxygen from the atmosphere.
As a result of a chemical reaction between the atoms of a given metal and oxygen, metal oxides are formed (the metal atom gives up an electron to oxygen, thus forming a bond between them). This phenomenon occurs instantaneously, the mere presence of pure metal and oxygen molecules is enough.
Depending on the porosity of the metal surface, the amount of oxide will vary. Porous iron will be heavily coated with iron oxide (rust), then this rust will "flake off", and then the oxygen will penetrate deeper into the structure of the iron, generating more rust, which will flake off even more, and so on. On the other hand, stainless steel is much less porous, it oxidizes on the surface, and this oxide layer effectively limits further oxygen penetration deeper into the material.
The strength of the bond between metal and oxygen atoms also depends on the type of element. Using some simplification, we can say that copper oxide ""holds stronger" than tin oxide, and nickel oxide "holds even stronger" than copper oxide. It is worth knowing that iron oxide or aluminum oxide is "even stronger", meaning that it is much more difficult to remove than nickel oxide.
Bottom line: metal oxides form very quickly. Their removal requires a sufficiently active flux, and not every type of oxide can be removed with a given flux.
Flux action
Flux has several important functions:
- Removal of metal oxides. Metal oxides prevent the formation of a proper solder joint, so they must be removed beforehand. The main role of the flux is therefore to convert the metal oxides into metal salts, which are then washed away by the molten solder. The phenomenon of oxide removal involves the reaction of the activator contained in the flux with the metal oxide in question, resulting in the formation of metal salts and water. Flux activation is achieved by heating the flux to the required temperature for a certain period of time.
- Protection against reoxidation. After removing the oxides, and before covering with liquid alloy, the soldered surface can be reoxidized. Therefore, it is necessary to apply enough flux so that a thin layer of it remains on the cleaned surface and protects it from reoxidation until it is filled with liquid solder. Protection against reoxidation is provided by rosin/resin or other solid material used in the flux.
- Removing contaminants. Flux also removes some other contaminants from surfaces, "washing" them away. Of course, not every type of contaminant can be removed by flux, so solder pads and wires should generally be clean.
- Reducing surface tension. Reducing the surface tension of the solder makes it easier to spread the liquid alloy and provides better wetting. This significantly reduces the wetting angle of the solder and improves the quality and appearance of the solder joint.
- Improved heat transfer. When soldering with a soldering iron (e.g., by hand), flux facilitates heat transfer from the soldering iron to the surfaces to be joined.
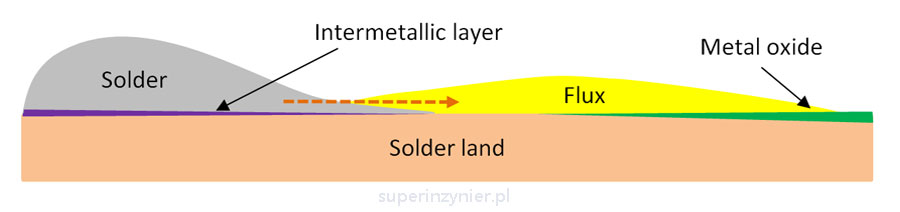
The following illustration shows an idea in which the flux removes metal oxides, allowing the liquid alloy to form a solder joint, i.e. to form an intermetallic phase. At the same time, the flux protects the cleaned surface from contact with oxygen in the area where the solder has not yet covered the soldered surface. The liquid solder alloy, while wetting the solder field, simultaneously "pushes" the flux, metal salts and contamination out of the area.
Flux chemical composition
The exact composition of fluxes is a know-how, so it is often a manufacturer's secret, so below is a general summary of the groups of materials used:
- Activator. It is the most important component of the flux. Its main role is to remove metal oxides. Activators can have different levels of acidity. They are usually organic acids, which is why you may encounter the term "weak organic acids" (WOA - Weak Organic Acids). Some activators may contain halides, which are a source of halide ions (usually Cl-, Br-).
- Solvent. The liquid in which the activator and solid components of the flux are diluted. If the solvent is isopropanol (IPA - Isopropanol Alcohol), then we say that the flux is "alcohol-based". If the solvent is water, the flux is "water-based" or "VOC-free". Solvents containing water and a small amount of alcohol are also found, in which case such fluxes are referred to as "Low-VOC".
- Solids, Vehicles. This is a material that protects against reoxidation of the metal surface by oxygen and facilitates the removal of contaminants from the soldering area. It is usually some form of rosin or synthetic resin, glycol, glycerin, etc.
- Additives. Manufacturers add various types of substances to improve coverage, reduce surface tension, etc.
J-STD-004 classification
The Global Electronics Association (IPC) has developed the J-STD-004 standard, which classifies fluxes according to composition, activity level and halide ion content[1].
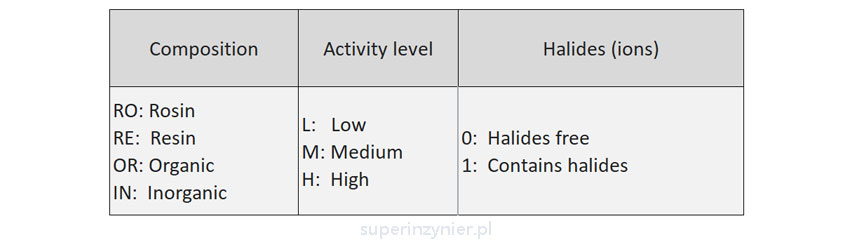
For example, a flux may have the designation REL1. What does this mean? RE is resin, L means low activity level, 1 means it contains halide ions.
Read more about each category:
Composition
- RO - Rosin (colophony). Rosin or colophony is a natural product that is extracted from the stumps or bark of pine trees. It is dark brown in color and leaves traces of this color on PCBs. It is not soluble in water. Any washing requires the use of appropriate chemicals.
- RE - Resin. This is a resin other than rosin. The color is usually lighter than dark brown, and the appearance of the residue varies depending on the type of resin.
- OR - Organic compounds. A material such as glycol. The residue is more transparent and "less visible" than resin or rosin. The board visually looks cleaner (we are talking about optical cleanliness), it has nothing to do with ionic cleanliness. Usually the material is water-soluble, so OR-based fluxes are often washable with warm water.
- IN - Inorganic compounds. Materials such as hydrochloric acid, hydrofluoric acid, stannous chloride, sodium or potassium fluoride, etc. Nowadays rarely used due to high corrosivity.
Activity level
The activity level is based on several criteria related to halide ion content and corrosion. These tests include surface resistance measurement (SIR), copper mirror test, electrochemical corrosion test (ECM), etc.
- L - Low.
- M - Medium.
- H - High.
Halides (ions)
Halides, commonly chlorine or bromine compounds, are frequently used in fluxes. This includes chlorides and bromides. Formally, fluorides and iodides are also considered halides. Their ions: Cl-, Br-, F-, I-, contribute to the removal of metal oxides during the soldering process, but unfortunately, they can degrade ionic purity. The J-STD-004 standard defines two classes for halide levels:
- 0 - Zero. Level less than 0.05% by weight, considered as the absence of halide ions.
- 1 - One. Contains halide ions.
MIL-F-14256 classification
MIL-F-14256 is an old American standard that was canceled in 1995 and replaced in the realm of fluxes by J-STD-004. However, the classification defined by this standard can still be found in literature and datasheets. Below is a brief overview of the main classes:
- R - Rosin. The mildest resin-based flux, free of halides.
- RMA - Rosin Mildly Activated. Resin-based flux containing activators, typically with a low level of halides. This represents a moderate level of activity and may require cleaning after soldering.
- RA - Rosin Activated. Resin-based flux containing activators and a significant amount of halides. Highly active, requires cleaning after soldering.
- WS - Water Soluble. Water-soluble flux, typically in an active form, meaning it requires cleaning after soldering.
ISO 9454-1 classification
The ISO 9454-1 standard classifies fluxes according to the following key:[2]
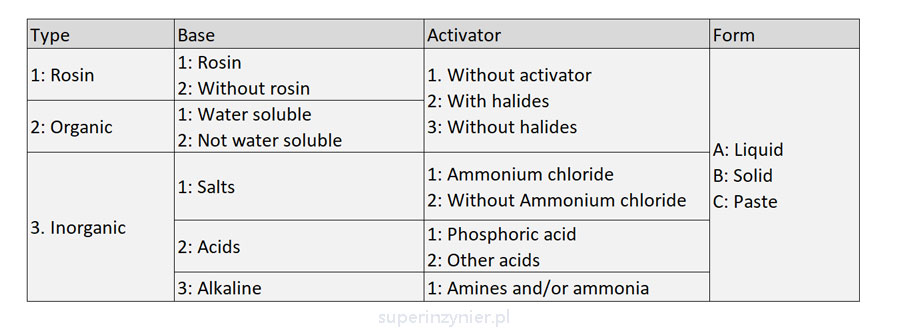
According to this classification, a flux that is liquid, colophony-based, and without activators will be designated as 1.1.1.A. A liquid, organic flux, insoluble in water with activators containing halide ions will be classified as 2.2.2.A.
DIN 8511 classification
DIN 8511 is an outdated German standard. The individual classes of fluxes were designated with the symbol F-SW-nn, where nn represents two digits indicating a specific flux class. The standard has been withdrawn, so it can be disregarded ;)
No-clean flux
This term refers to fluxes whose residues may not require cleaning. Limiting or eliminating the PCB cleaning process undoubtedly has a positive impact on the environment and reduces production costs.
Depending on the PCB project and application, residues of no-clean flux may be permissible and left on the PCB surface or must be removed. Additionally, a no-clean flux that can be left on the PCB must be properly cured in the soldering process, should not be tacky, should not spread onto connector contact surfaces, etc.
Clean flux
Fluxes referred to as clean are chemical agents with a high level of activity, greatly facilitating the soldering process. Residues from these fluxes can lead to a decrease in Surface Insulation Resistance (SIR) on the PCB and ultimately to electrochemical migration. For this reason, flux residues must be removed after soldering, so assembled boards must be cleaned. Cleaning is not a straightforward procedure, and depending on the PCB project, it may sometimes be very challenging or even impossible to correctly remove residues.
Ionic cleanliness
Residues of flux on the surface of PCBs (after the soldering process) can be categorized into solid particles such as colophony, which are visible, and ionic contaminants, typically invisible in optical inspection.
Residues of colophony or other resins typically do not affect the operation of electronics; they are merely a "visual aspect."
The problem lies with ionic contaminants. Under the influence of the electric field, moisture, and these ions on the surface of the PCB, there is a decrease in surface resistance and, ultimately, the occurrence of electrochemical migration. If electrical connectors are contaminated, then contact corrosion may occur.
The application of the proper soldering profile is crucial for ionic cleanliness. Read the article on reflow soldering profile and wave soldering profile.
Standards and the assessment of ionic cleanliness, determining appropriate limits, constitute a highly complex and challenging topic. I will provide more information in a dedicated article.
Summary
Fluxes are a crucial chemical used in the soldering process and are available in liquid, gel, or as a component of SMT solder paste.
The proper selection of flux for a given application, correct usage (quantity, time-temperature profile), and monitoring of ionic cleanliness levels are key aspects that determine high-quality solder joints and appropriate ionic cleanliness. It's important to note that "no-clean" flux does not always mean "no cleaning"; much depends on the PCB design and the working environment of the manufactured device.
References
- IPC J-STD-004
- ISO 9454-1